mRNA-1273
COVID-19 Vaccine Development
Moderna's groundbreaking coronavirus vaccine was designed in just 2 days by Susie Neilson , Andrew Dunn , and Aria Bendix Dec 19, 2020
The Food and Drug Administration granted emergency authorization to Moderna's coronavirus vaccine on Friday.
The decision came after an independent expert panel voted overwhelmingly to recommend the authorization on Thursday. Moderna's vaccine candidate was found to be 94.1% effective in preventing COVID-19 in clinical trials, and it doesn't trigger severe side effects in most people.
That's far more effective than expected: The FDA had said it would likely approve a vaccine that showed at least 50% efficacy, and Dr.Anthony Fauci had said he hoped for 70%. The vaccine's development process was also unprecedentedly fast — only the Pfizer-BioNTech team beat Moderna to FDA authorization (that vaccine, similarly, was 95% effective in trials).
But perhaps more remarkable is that Moderna designed its vaccine in just two days in January, before some people had even heard of the coronavirus.
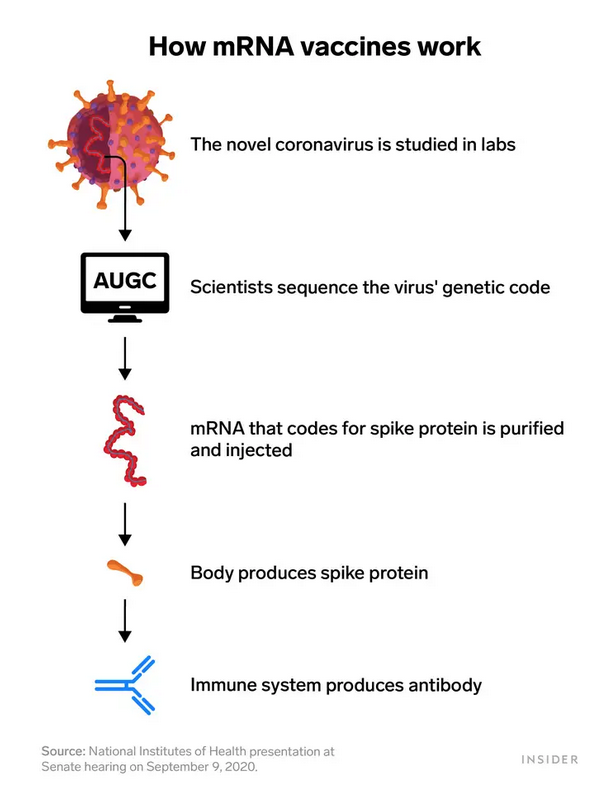
Utilizing mRNA technology meant that both Pfizer and Moderna only needed the coronavirus' genetic sequence to make a vaccine — no virus had to be cultivated in labs. That's why the companies were able to progress in record time. By contrast, the development of more traditional vaccines can take years.1)
Specifications & Regulations
Moderna's mRNA-1273 injectable questionably vaccinish thing…
Emergency Use Authorization Fact Sheet for Recipients and Caregivers
Moderna Vaccine Recall
Moderna Recalls 764,900 COVID-19 Vaccine Doses After Contamination Found Epoch Times By Lorenz Duchamps April 9, 2022
The U.S. pharmaceutical and biotechnology company Moderna Inc. on Friday issued a recall in Europe involving 764,900 doses of its COVID-19 vaccine “Spikevax” after contaminants were discovered in a vial.
“The lot is being recalled due to a foreign body being found in one vial in the lot manufactured at the company’s contract manufacturing site, ROVI,” Moderna and Spain’s ROVI Pharma Industrial Services said in a joint statement.
The drugmaker did not specify what kind of foreign substance was found and had recalled the whole lot out of “an abundance of caution.”
The contamination was traced in just one vial of the batch and investigators do not believe the contamination posed a risk to other vials in the lot.
“Moderna conducted a cumulative search of its global safety database, and no safety concerns were reported in individuals who received the Moderna COVID-19 vaccine from this lot. To date, no safety or efficacy issues have been identified,” according to the statement.
The lots were distributed from Jan. 13 to Jan. 14 in Norway, Poland, Portugal, Spain, and Sweden. To date, more than 900 million doses of the Moderna COVID-19 vaccine have been administered worldwide.
Last year, Moderna had several lots of its COVID-19 vaccines recalled by Japanese authorities after an investigation found stainless steel contaminants in some vials. The recalled batches were manufactured by the same Spanish company, ROVI.
Japan’s biggest drugmaker, Takeda Pharmaceutical, said in a statement the contamination was traced back to the production run by ROVI. The findings were discovered by an investigation carried out by the two companies, not the Japanese health ministry.
Three men in Japan had fallen severely ill in August 2021 after being administered a second dose of the now-recalled COVID-19 vaccine and died shortly after. Takeda said in a statement at the time there is no evidence they are linked to the vaccine, Reuters reported.
“Stainless steel is routinely used in heart valves, joint replacements, and metal sutures and staples. As such, it is not expected that injection of the particles identified in these lots in Japan would result in increased medical risk,” the company said.
The first two deaths reported in the country linked to contaminated Moderna doses were two men, aged 30 and 38. They both died two days after receiving a second dose from a tainted batch of vaccines.
The third case was a 49-year-old man, who also fell ill after receiving his second dose, and died the next day, the health ministry said, noting that his only known health issue was a buckwheat allergy.2)
Moderna Vaccine Videos
A Moderna representative admits to a caller that she, and all who take their mRNA “vaccine” (ie. gene therapy), are still part of a clinical trial with unknown long term effects. Scientists have been trying to warn the public of these risks for over a year — only to be censored. 3)
Moderna FOIA
Contributed by Bob Herman (Axios) 4)

