Anthrax Vaccines
Anthrax bioweapons background
“Anthrax was first tested as a biological warfare agent by Unit 731 of the Japanese Kwantung Army in Manchuria during the 1930s; some of this testing involved intentional infection of prisoners of war, thousands of whom died.
Anthrax, designated at the time as Agent N, was also investigated by the allies in the 1940s. The British army tested experimental anthrax weapons on Gruinard Island, off the northwest coast of Scotland, in 1943. Gruinard was burned over at least once, yet as of the late 1980s, it was still too heavily contaminated with spores to allow unprotected human access, indicating the hardiness of anthrax spores. Weaponized anthrax was part of the U.S. stockpile prior to its destruction in 1972.” 1)
Anthrax Vaccine History
The original anthrax vaccine in the United States was developed by George Wright and others in the 1950s and was first produced on a large scale by the pharmaceutical manufacturer Merck Sharp & Dohme. A clinical study in 1962 evaluated the safety and effectiveness of the Merck vaccine in mill workers. This study formed the basis for subsequent licensure of a modified vaccine in 1970. The Division of Biologics of the National Institutes of Health (NIH) issued the original license for anthrax vaccine to the Michigan Department of Public Health. In 1995, the facility changed its name to the Michigan Biologic Products Institute. In 1998, the facility was sold, and its name was changed to BioPort Corporation.
Over time, FDA has cited the facility for repeated deviations from applicable manufacturing standards for the vaccine. The facility received warning letters from FDA, including one in March 1997 stating its intent to revoke the facility’s license.
Merck Sharp & Dhome is a subsidiary of Merck & Co.Inc.2)
In the original formula (which had FDA approval), two principal ingredients (formaldehyde and benzethonium chloride) have not been evaluated for human consumption. Actually, The FDA never tested the state's vaccine for human consumption. The license issued to the state lab was based on evaluation of a sample made by another manufacturer (Merke, Sharpe and Dohme) The vaccine is only effective against cutaneous (skin contact) anthrax. It has never been tested against inhaled anthrax (the deadliest form that would be used in biological weapons). 3)
*March 16, 1999* Senate Appropriations Committee Prepared Statement by Robert C. Myers, *DVM* Chief Operating Officer and Director, BioPort Corporation,4)
Whitney Webb September 11, 2020.
One of the most politically-connected yet scandal ridden vaccine companies in the United States, with troubling ties to the 2001 anthrax attacks and opioid crisis, is set to profit handsomely from the current Coronavirus crisis.
August 2001 biopharmaceutical company BioPort faced imminent disaster. A series of company scandals, controversial federal bail-outs and severe, adverse health reactions among U.S. troops were causing both Congress and the Pentagon to reconsider its multi-million dollar contract to provide the military with an anthrax vaccine.
Formed for the sole purpose of acquiring a publicly-owned company in Michigan that held the exclusive license to manufacture the only FDA-approved anthrax vaccine in the United States, BioPort sought to quickly expand the size and scope of its contracts with the U.S. military. This strategy was made possible thanks to the former head of the Joints Chiefs of Staff, Adm. William Crowe, who would prove highly instrumental in the rise of BioPort’s vaccine monopoly and its subsequent, aggressive hiring of former government officials as lobbyists.
One of their biggest proponents of expanding BioPort’s contracts was working for HHS at the time — Jerome Hauer, a man who not only had foreknowledge of the anthrax attacks, but had also participated in the Dark Winter simulation that would also predict those same attacks just months prior. Hauer would, months later, be appointed to a newly created position at HHS, one which oversaw the new biodefense stockpilefrom which BioPort would be a major beneficiary.
BioPort would be then renamed and repackaged as Emergent Biosolutions in 2004. It would then hire even more well-connected lobbyists and add several big names from government and the private sector to its board. One of these “big names” was none other than Jerome Hauer, who was added to Emergent’s board soon after leaving HHS. Hauer still remains a company director and sits on three of its corporate governance committees
The clear nexus between Big Pharma, Government and University-affiliated Biosecurity Centers offers a startling look into the Biotech-Industrial Complex that has long dominated U.S. biodefense policy and is now guiding much of the U.S. government’s response to the Coronavirus crisis 5)
Anthrax Vaccine News Timeline
Important Note - BioPort Labs, long sole source for anthrax vaccines was renamed Emergent Biosolutions known for its backdoor GoF Gain of Function contract with Wuhan Institute of Virology by Peter Daszak and Anthony Fauci
Feb 18, 1996 FCW Federal Contractors Weekly - Mitre spinoff targets broader client base. By John Monroe
Mitre Corp. has spun off a new company called Mitretek Systems Inc which will seek information systems engineering projects among agencies outside its primary client base in the Defense Department and the Federal Aviation Administration.
The creation of Mitretek, announced earlier this month, reflects DOD's new policy of limiting Federally Funded Research and Development Centers FFRDC to what it defines as the “core workload”
Mitretek will not compete for contracts against commercial companies. The company will negotiate sole-source contracts with agencies as Mitre has done. Mitretek, operating outside constraints placed on DOD FFRDCs will be able to diversify its business, Horowitz said. 6)
*December 15, 1997* Secretary of Defense William Cohen approved the Anthrax Vaccine Immunization
Program AVIP a plan to immunize the Total Force against anthrax.
At the request of DoD, the Department of Health and Human Services convened a civilian panel, the *Anthrax Vaccine Expert Committee* AVEC, to review each VAERS report submitted for the anthrax vaccine. After two years, in which almost 1,200 reports and medical records have been reviewed, the AVEC continues to report that they have identified no unexpected events and no disease syndromes associated with the anthrax vaccine. More than 487,000 Service members have received over 1.9 million vaccinations and are today benefiting from this protection. pages 107-108.7)
March 12, 1999 Los Angeles Times By PAUL RICHTER Sailors Punished Over Anthrax Shots
WASHINGTON — Twenty-nine sailors aboard the aircraft carrier Theodore Roosevelt have been punished for refusing to accept anthrax vaccine shots, the Navy announced Thursday, in the latest sign of resistance in the ranks to what Pentagon officials believe is a low-risk preventive measure.
The Navy said that the 29 service members on the Norfolk-based nuclear carrier have been demoted, fined, jailed for up to 45 days or given extra duty for refusing to undergo the mandatory round of six injections to protect them from the deadly biological warfare agent.
Since the Pentagon began administering the injections last year, about 100 military service members have refused orders to take shots that the Pentagon considers necessary because of the increasing chance of a germ-warfare attack.8) 9)
August 27, 1999 Robert F. Dorr , Air Force Times
On Sept. 18, members of the Air National Guard at Stewart Field near Newburgh, N.Y., are scheduled to begin receiving anthrax inoculations.
One member who isn't planning on standing in line for the shots is Tech. Sgt. William F. “Bill” Mangieri, 30, a readiness technician in the 105th Airlift Wing. Mangieri's job is to train others for nuclear, biological and chemical warfare.
“I am concerned about the safety and effectiveness of the vaccine,” Mangieri told me in an Aug. 14 telephone interview. “I also question whether we need it. I'm anticipating a mass walkout at military bases over this issue,” he added. I'm worried that we're not going to have enough pilots and maintenance people….
A bill in the House of Representatives, co-sponsored by Reps. Benjamin Gilman and Sue Kelly, both New York Republicans, would halt all anthrax vaccinations pending the result of a new investigation. Another bill, introduced by Rep. Walter Jones, R-N.C., would let members choose whether to be immunized.
Defense Secretary William S. Cohen has warned that, “We should expect more countries and terrorist groups to seek, and to use, [biological] weapons.” Cohen's supporters view him as a visionary, delivering a wake-up call about a frightening new world in which fanatical adversaries wield horror weapons.“We know we have a safe, effective vaccine. We would be irresponsible not to use it,” said Virginia Stephanakis, a Pentagon spokeswoman.
Cohen's detractors include Milton Leitenberg, senior fellow at the Center for International and Security Studies at the University of Maryland, who has accused Cohen of having “fantasies.” In an Aug. 14 letter to The Washington Post, Leitenberg said of the prospect of an anthrax attack, “No agency of the U.S. government has prepared a threat analysis that provides indications that these events are imminent or even likely.”
The program to vaccinate all 2.4 million military troops against anthrax has run into delays attributed to the only company that produces the vaccine.“ 10)
*September 28, 1999* Maxygen Announces $6.7 Million Grant from DARPA to Develop Aerosolized Vaccines
Maxygen, Inc. (Nasdaq: MAXY) announced today that it has received a $6.7 million three-year grant from the Defense Advanced Research Agency (DARPA) to use its proprietary MolecularBreeding™ directed evolution technology (also known as “DNA Shuffling”) to develop aerosol-based vaccines to protect against a broad spectrum of pathogens.
Aerosol delivery of prophylactic and therapeutic agents is potentially the simplest and most cost-effective way to protect or treat humans and animals from disease epidemics. In addition to this recent DARPA grant, Maxygen is the recipient of two additional DARPA grants in the vaccine area. In February 1998, Maxygen received a $5.6 million grant to evolve novel DNA vectors for efficient delivery and expression of pathogen antigens, and in April 1999, the Company received a grant of $7.7 million to generate novel vaccine antigens for a wide range of pathogens.
Maxygen, Inc., headquartered in Redwood City, CA is a private biotechnology company that is focused on the development of novel and improved commercial products using its proprietary MolecularBreeding™ directed evolution technology for a variety of industries including human therapeutics, chemicals, vaccines, industrial enzymes, and agriculture. The Company is the leader in nucleic acid recombination technologies for imparting new properties into single genes, multi-gene systems, pathways, vectors and genomes. Maxygen has commercial relationships with several companies including Novo Nordisk in certain areas of industrial enzymes, Dupont Pioneer Hi-Bred and ZenecaAgrochemicals Astra Zeneca for specific products in agriculture and DSM Anti-Infectives for improved manufacturing of certain classes of penicillin antibiotics.
Maxygen was founded in a March 1997 spinout from Glaxo Wellcome-Affymax, led by biotech entrepreneur Alejandro Zaffaroni, and MolecularBreeding™ inventor Pim Stemmer. 11)
Military - Industrial - PhARMA -anthrax threat and remedies
December 4, 2001 PBS - Betty Ann Bowser
Officials are taking another look at the controversial anthrax vaccine
Secretary of Defense Donald Rumsfeld said he was hopeful the vaccine program could be salvaged. *”We're going to try to save it, and try to fashion some sort of an arrangement whereby we give one more crack at getting the job done with that outfit.* It's the only outfit in this country that has anything under way, and it's not very well under way, as you point out.“ Rumsfeld 13)
September 2002 - Report to Congress
ANTHRAX VACCINE GAO’s Survey of Guard and Reserve Pilots and Aircrew
Most of the information in this report was derived from the results and analyses of survey questionnaire responses received from selected pilots and other aircrew members of the Air National Guard and the Air Force Reserve before the events of September and October 2001.
December 1997, the Secretary of Defense announced a plan to inoculate U.S. forces against the potential battlefield use of anthrax as a biological warfare (BW) agent. The mandatory AVIP—using the only available vaccine produced by the BioPort Corporation—was officially launched in August 1998 as a high-priority commander’s program. This means that unlike other mandatory vaccines routinely given to the military, AVIP received intense attention from high command levels and was subject to exceptional accountability requirements. It was intended to be compulsory for all 2.4 million DOD military service members—active duty and reserve component members, including certain designated civilian and contractor personnel. DOD still regards the biological agent anthrax, a disease that is usually lethal if inhaled in sufficient quantity, as the single greatest BW threat to U.S. military forces in the battlefield. 14)
*2002* The FDA's new label (2002) on the anthrax vaccine admits to a systemic averse reaction rate of between 5% and 35% - whereas previously, the DoD claimed it was a mere .02%. Since then, the GAO (Government Accounting Office, a watch-dog agency) has come out with a new report estimating the systemic adverse reaction rate is probably as high as 85%. In addition, it is known that women have twice the adverse reaction rate as men.15)
November 8, 2002 CIDRAP News - Center for Infectious Disease Research and Policy and GAO: Military anthrax shots caused many reactions, prompted some pilots to quit
The Pentagon's mandatory anthrax shots caused adverse reactions in most recipients and helped prompt many Air Force Reserve and Air National Guard members to transfer to other units or leave the military between 1998 and 2000, according to a survey by Congress's General Accounting Office (GAO).
The survey indicated that 85% of troops who received an anthrax shot had an adverse reaction, a rate far higher than the 30% claimed by the manufacturer in 2000, when the survey was conducted. Sixteen percent of the survey respondents had either left the military or changed their status, at least in part because of the vaccination program.16)
July 7, 2003.IRVING, Texas —Carrington Laboratories, Inc (Nasdaq: CARN) today announced that Dr. Ronald Ray Blanck, a retired lieutenant general and former Surgeon General of the U.S. Army and commanding general of the Army Medical Command, has been elected to the company’s board of directors. Dr. Blanck, 61, who is currently president of the UNT Health Science Center (UNTHSC) in Fort Worth, joins the existing six-member board. He assumed his current position at the UNTHSC shortly after retiring from the Army in July 2000.
DelSite Biotechnologies, Inc a wholly owned subsidiary of Carrington, is an early stage biotechnology company established to commercialize its novel polymer drug delivery technology. Currently, DelSite is focusing its resources on developing delivery systems for vaccines and therapeutic proteins and peptides that could benefit from improved injectable, intranasal and topical routes of administration.
Carrington’s wholly owned subsidiary, Sabila Industrial, S.A. is the company’s bulk pharmaceutical manufacturing facility in Liberia, Costa Rica. The ISO 9000, USDA and FDA licensed manufacturing facility produces the company’s proprietary raw materials 17)
August 19, 2003 LINCOLNSHIRE, Ill.–(BUSINESS WIRE)
BioSante Pharmaceuticals, Inc. (OTC BB:BISP) announced today results of several studies demonstrating the superiority of its innovative calcium phosphate nanoparticulate CAP vaccine adjuvant and delivery system, BioVant(TM), compared to the only approved adjuvant, aluminum salts (alum).
This announcement is from a Mitretek press release. The Board of Trustees of Mitretek Systems, Inc., a non-profit research engineering organization, have elected Ronald R. Blanck, former Surgeon General of the U.S. Army and commander of the U.S. Army Medical Command, to serve as a member of the Board. Commentary: Retired LTG (Dr.) Ronald Blanck has already been paid as an “expert” by BioPort for a junk-science report advocating use of BioPort's anthrax vaccine for American civilians. Now Blanck is receiving the payoff for repeatedly endorsing the junk-science supplemental testing of BioPort's anthrax vaccine stockpile in 1998-2000. 18)
*2008 December 19*
ROCKVILLE, Md., Dec 19, 2008 (BUSINESS WIRE) – Emergent BioSolutions Inc. (NYSE:EBS) announced today that the U.S. Food and Drug Administration (FDA) has approved Emergent's supplemental Biologics License Application (sBLA) for Anthrax Vaccine Adsorbed (BioThrax(R)), the only FDA-licensed vaccine to prevent disease caused by Bacillus anthracis. The supplement provides for a change in the route of administration and a reduction in the total number of vaccinations. The new schedule for BioThrax is five intramuscular (IM) doses at 0, 1, 6, 12 and 18 months, compared with the former schedule of six subcutaneous (SC) doses at 0, 2 weeks and 1, 6, 12, 18 months.19)
*2003 December22*
Just eight days after the Dec. 22, 2003 decision by federal Judge Emmett Sullivan's that orders to take the vaccine were illegal, the FDA issued a ruling that it was both legal and safe - based on animal data. See further developments in the lawsuits section. 20)
May 26, 2004 Carol D. Leonnig, Washington Post Judge Sees Little Evidence To Support Anthrax Vaccine
A federal judge said yesterday he had significant doubts about whether the federal government has enough scientific evidence to show that the anthrax vaccine required for military personnel is either safe or effective.
U.S. District Judge Emmet G. Sullivan, who will decide in coming weeks whether to halt the Defense Department's mandatory anthrax inoculations, also criticized the government's review of the vaccine as “one of the most jumbled, confusing” processes he had ever seen. 21)
*July 21, 2004* The Office of Public Health Emergency Preparedness at DHHS, formed after the2001 anthrax attacks, began to coordinate civilian medical countermeasure development by the National Institute of Allergy and Infectious Diseases, CDC, and the Department of Defense, under the leadership of eminent scientists and physicians such as DA Henderson and Philip K Russell. On July 2004, President Bush signed legislation creating Project Bioshield, a $6 billion,10-year program for acquiring new medical countermeasures for the Strategic National Stockpile.
Furthermore, DHHS has planned for the stockpiling of the licensed anthrax vaccine, a new recombinant anthrax vaccine, more doses of botulinum antitoxins, a safer smallpox vaccine that can be given to immunocompromised individuals, and anthrax adjunctive therapies. page 39 22)
*February 21, 2007*. by Michael Hardy Washington Technology
Mitretek Systems has *changed its name* to Noblis Inc., a move that company officials believe better reflects the 11-year-old company's nature. Noblis, a non-profit science, technology and strategy company, positions itself as free of commercial interests and vendor alliances.
Noblis of Falls Church, Va., specializes in systems, process and infrastructure problems in fields including transportation, health care, homeland security, risk assessment and other fields. 23)
*2007 Corporate News *
Emergent Biosolutions Inc at Biotechnology Industry Organization Investor Forum
Emergent BioSolutions Inc. is a biopharmaceutical company dedicated to one simple mission—to protect life. We develop, manufacture and commercialize immunobiotics, consisting of vaccines and therapeutics that assist the body’s immune system to prevent or treat disease. Our biodefense business focuses on immunobiotics for use against biological agents that are *potential weapons of bioterrorism and biowarfare*. Our marketed product, BioThrax® Anthrax Vaccine Adsorbed, is the only vaccine approved by the U.S. Food and Drug Administration for the prevention of anthrax infection. Our commercial business focuses on immunobiotics for use against infectious diseases and other medical conditions that have resulted in significant unmet or underserved public health needs. 24)
2008 Corporate News
ROCKVILLE, Md.–(BUSINESS WIRE)–Emergent BioSolutions Inc. (NYSE: EBS - News) announced today that the final analysis from a recently completed, randomized, placebo-controlled, blinded Phase II clinical study reaffirmed that its single-dose, drinkable typhoid vaccine candidate was highly immunogenic and well-tolerated with an acceptable safety profile in the population studied. For the study, a total of 151 Vietnamese children between 5 and 14 years of age were enrolled. A total of 101 children received the vaccine candidate and 50 children received placebo. This clinical study is the first trial involving a pediatric population and was performed in collaboration with the Wellcome Trust, Oxford University and the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
“We are pleased that the full analysis of the data from this Phase II study reaffirms that our typhoid vaccine candidate met the pre-established clinical endpoints. These data are encouraging and indicate great promise regarding both the safety and efficacy of what would be the first single-dose, drinkable typhoid vaccine,” stated Fuad El-Hibri, chairman and chief executive officer of Emergent BioSolutions. 25)
War on Terror & Biological Warfare
December 19, 2001 Rense By Ian Gurney- Vaccine-Maker BioPort And bin Laden - A Profitable Symbiosis?
(select quotes - link to full article)
According to a story published by the Pakistan News Service on the 1st of December, documents referring to US anthrax vaccine-maker BioPort Inc. were found in the possession of the al-Qaeda in Kabul, Afghanistan.
The documents came from the US Department of Defence, but were not classified and contained highlighted items and stars scribbled across the top of one page. A BioPort spokeswoman Kim Brennen Root commented: “The discovery supports the notion that the al-Qaeda and the Taliban have been studying biological warfare and protecting against weapons of mass destruction.
BioPort is the only corporation in the United States with a license to make the Anthrax vaccine. Except that BioPort doesn't actually make the vaccine, BioPort simply bought the laboratory that does make the vaccine, Michigan Biologic Products Institute, from the State of Michigan in 1998. Less than a month after it took over the business from the state-owned Michigan Biologic Products Institute in September 1998, along with the actual Anthrax vaccine, BioPort acquired Michigan Biologic Products Institute's sole and exclusive customer for the vaccine, the US Department of Defence, and an exclusive $29 million contract with the Department of Defence to “manufacture, test, bottle and store the anthrax vaccine.”
According to former [CIA] Central Intelligence Agency military analyst Patrick Eddington, the estimated $60 million worth of anthrax vaccine Bioport is expected to produce for the Defence Department over the next five years could just be the beginning.
The Pentagon has a $322 million, 10-year program to develop at least three, and perhaps as many as a dozen additional biological warfare vaccines,î Eddington told ABCNEWS. However, since acquiring Michigan Biologic Products Institute, BioPort has only delivered a small amount of the anthrax vaccine to the US Defence department. In fact, just 4% of the vaccine contracted for has so far been delivered because Federal Drug Administration FDA audits have uncovered suspicious record keeping as well as security and contamination problems at BioPort's North Lansing laboratory, causing the FDA to ban delivery of the product…
So, now lets look at who owns and runs BioPort Corporation. Let's begin with Admiral William J. Crowe, Jr. former Head of the US Joint Chiefs of Staff and a former US ambassador to Great Britain. It seems that back when President George H. W. Bush was setting up Osama Bin Laden as a “freedom fighter” (Afghanistan's “freedom fighters” were credited with shooting down more than 270 Soviet aircraft using American made Stinger missiles in the 1980's) the good Admiral and his associates on the Joint Chiefs of Staff were, according to some reports, selling American made weapons-grade Anthrax to Saddam Hussein in the hopes that he would use it against Iran…
Rumours, denied by BioPort, have suggested that one investor in the company is the Carlyle Group, one of America's most successful investment companies, well known for generating “extraordinary returns” for its customers through investments in the US defence industry.” 26)
Biowarfare Propaganda & Emergency Powers
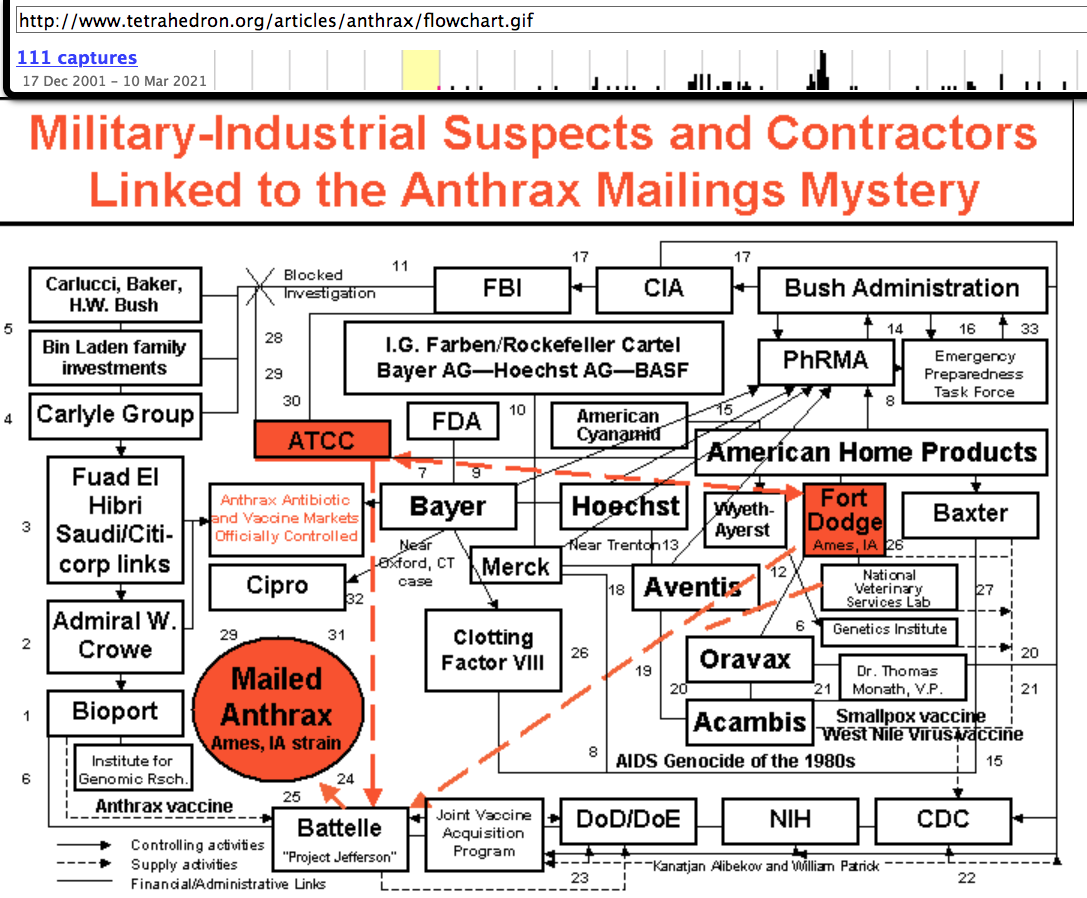
2001 December - Could the Anthrax Mailings Be Military-Industrial Espionage?
A Special Report By Leonard G. Horowitz, D.M.D., M.A., M.P.H.
Broadcasters such as Peter Jennings on the ABC Nightly News September 26, 2001 featured the Bayer pharmaceutical company's anthrax antibiotic Cipro, without mention of far less costly and risky alternatives to mass population prophylaxis. The Food and Drug Administration [FDA] and Centers for Disease Control and Prevention [CDC] advanced Cipro for anthrax along with a “Model State Emergency Health Powers Act” for generic bioterrorism response including forced drugging and certain vaccinations for anthrax and smallpox.(2)
Due to the panic and official endorsements, Cipro sales skyrocketed more than 1000%.
At a single consumer prescription cost of $700 for a mere sixty-day supply, the resulting revenue helped rescue Bayer from the brink of bankruptcy.(3) Then, Department of Health and Human Services HHS Secretary Tommy Thompson rushed to spend millions more on a “special” Cipro contract in further defense of the nation's health.(4) By November, in the wake of the anthrax mailings, more than 30,000 panicked people in the United States were taking Cipro.(5) Unreported by the media was the grave likelihood that given Cipro's myriad side effects, and largely untested status, more people had died from taking the drug than the handful who had succumbed from the anthrax mailings.
The Washington Post (March 16, 1999), science policy analyst Daniel S. Greenberg criticized what he called *a whiff of hysteria-fanning and budget opportunism in the scary scenarios of the saviors who have stepped forward against the menace of bioterrorism While a gullible press echoes [their] frightening warnings, there are no independent assessments* of the potential for terrorist attacks or the practicality of the proposed responses. 27)
Gulf War Syndrome Effects Study Anthrax
Anthrax and Gulf War Illness (GWI): Evidence for the Presence of Harmful Anthrax Antigen PA63 In the Serum of Veterans with GWI
Effie-Photini C. Tsilibary1,2, Eric P. Souto1, Marian Kratzke1,2, Lisa M. James1,2,3, Brian E. Engdahl1,2,4, Apostolos P. Georgopoulos1,2,3,5* Brain Sciences Center, Department of Veterans Affairs Health Care System, Minneapolis, Minnesota, USA Department of Neuroscience, University of Minnesota Medical School, Minneapolis, Minnesota, USA Department of Psychiatry, University of Minnesota Medical School, Minneapolis, Minnesota, USA Department of Psychology, University of Minnesota, Minneapolis, Minnesota, USA Department of Neurology, University of Minnesota Medical School, Minneapolis, Minnesota, USA 28)
Gulf War Syndrome and Anthrax Vaccines
The Anthrax Vaccine: Making Our Soldiers Guinea Pigs by Kathleen Deoul | March 22, 2016
In 1965, the Department of Defense and the Centers for Disease Control awarded MBPI a contract to initiate development of an anthrax vaccine. MBPI received a license from the FDA to produce the vaccine in 1970.
Since anthrax had been virtually eliminated in the United States, only about 68,000 doses of MBPI’s anthrax vaccine were administered over the next two decades an average of about 1,300 per year. It was a marginal product used primarily by veterinarians and tannery workers. The Iraqi invasion of Kuwait would radically alter its status.
As the U.S.-led coalition deployed to the Persian Gulf in 1990, military commanders were gravely concerned over the possibility that Saddam Hussein would attack with biological weapons. Since he was believed to hold substantial stockpiles of anthrax spores, protecting our troops against this deadly disease was a high priority, and MBPI was the only vaccine manufacturer that had a licensed anthrax vaccine at that time. Even though it had not been approved for protection against inhalation anthrax, a decision was made to vaccinate all U.S. troops, and some 600,000 doses were administered – despite the fact that the vaccine had NEVER been tested for inhalation anthrax.
Department of Defense continues to insist that the anthrax vaccine is safe. DOD officials responsible for the Anthrax Vaccination Implementation Program point to recent reports by the National Institutes of Medicine’s Institute of Medicine (IOM) to back up their claim. Yet, these documents are far from the ringing endorsement the DOD officials would have the public believe. Indeed, the first IOM report, issued on March 30, 2000 concluded that:
“… in the peer-reviewed literature, there is inadequate/insufficient evidence to determine whether an association does or does not exist between anthrax vaccination and long-term adverse human outcomes.”
In another section the same report said -
“There is a paucity of published peer-reviewed literature on the safety of the anthrax vaccine.”
The IOM further noted that much of the research on the anthrax vaccine was conducted on animals. The report stated flatly that:
“Few meaningful conclusions regarding adverse effects in humans can be drawn from animal studies of the vaccine…”
The concerns about the lack of data voiced by the IOM echoed those expressed a six months earlier at an October 1999 congressional hearing.
One important witness was Dr. Kwai Chan, Director of the Government Accounting Office Special Studies and Evaluation Group. At that October 1999 hearing – over three years ago mind you – he told members of the House Government Reform Committee that:
“The long-term safety of the vaccine has not yet been studied.” The IOM report first discusses a study of textile mill workers conducted in 1962 and a 1985 CDC study holding them up as proof that the vaccine is effective. It then goes on to state, however, that:
“The small number of inhalation cases in those studies provides insufficient information to establish the vaccine’s efficacy against inhalational infection, but the data suggest the vaccine has a protective effect.”
They also base their finding on what they term “… reasonable assumptions of analogy…” between human and animal testing. It is well established in medicine, however, that there is no guarantee that results achieved in animals will be duplicated in humans. To imply that animal data could readily be applied to humans is at best highly questionable.
Yet another troubling pronouncement is the assertion that the vaccine would be effective against “… any known or plausible engineered strains of B. anthracis.” Again, no data is presented to support this astounding assertion.
What the researchers are trying to sugar coat is a systematic suppression of adverse event reports concerning adverse reactions to the anthrax vaccine that permeates the military medical service. Service members have complained that when they attempted to report vaccine reactions they were ignored, accused of malingering, or simply threatened to prevent them from submitting the information.
In fact, the situation generated so many complaints to Congress that in October of 2000, the surgeons general of all three military services issued instructions to their medical care-givers to take complaints concerning vaccine reactions seriously and to report them promptly.
Indeed, data that is contained in the IOM report indicate that anywhere from 60 percent to 70 percent of the vaccine-related adverse reactions were never reported.
According to GAO’s investigation, fully 77 percent of the guard and reserve pilots and aircrew members would not have taken the anthrax vaccine voluntarily. Nine out of ten said they were concerned about its safety. Two thirds said they did not support the DOD mandatory anthrax vaccination program.
Perhaps the most disturbing aspect of all, though, is that the rate of systemic reactions discovered by GAO was 100 TIMES as high as that claimed by the manufacturer!
According to the Department of Veterans Affairs, As of April 2002, 198,716 veterans of the Gulf War have filed claims for disability status for one or more conditions. That amounts to 28 percent of all that served! Of the claims filed, up to that point, 151,031 had been granted. This compares with a disability rate of 8.6 percent for World War II, 5 percent for the Korean Conflict and 9.6 percent for the Vietnam War.
The study, which was published in the respected British Medical Journal, compared the health outcomes of veterans of the Gulf War with those who performed peacekeeping duties in Bosnia. Not only did the study find a significant difference between the veterans who served in the Gulf and those who served in Bosnia, but they also found a particularly high incidence of so-called “multi-symptom illness,” what we now know as Gulf War Syndrome. Most important, the study directly linked this higher occurrence to the vaccines the soldiers received!
DOD also conducted a number of animal experiments on vaccines that contained a squalene adjuvant covering a wide range of diseases including toxic shock, malaria and, most importantly, anthrax!
The National Institute of Allergy and Infectious Disease (NIAID) has also sponsored human trials for vaccines using squalene as an adjuvant. In fact, such research began as early as 1987. Through 1999, the NIAID had conducted at least 23 trials of vaccines with a squalene adjuvant involving almost 1,200 human subjects!
Still, DOD officials have consistently insisted that they did not administer vaccines containing squalene to troops deployed to the Persian Gulf. Yet, GAO investigators found that this assertion, too, did not exactly square with the facts.
A study conducted at Tulane University on the presence of antibodies to squalene revealed a deeply disturbing pattern. The study examined blood samples from 144 Gulf War era veterans or military employees, 48 blood donors, 40 patients with systemic lupus erythematosus (SLE), 34 patients with silicone breast implants and 30 patients with chronic fatigue syndrome.
The study employed a “blinded” test – the gold standard in research – to determine if squalene antibodies were present.
What the researchers found was that 95percent of the Gulf War veterans suffering from chronic fatigue syndrome had antibodies to squalene, but that none of the Gulf War veterans who were healthy did. 29)
*VACCINE EPIDEMIC A Tragic History of Lies, Fraud, and Death Remains the Standard*
June 1, 2004 Mike Rupert's From The Wilderness - by Michael Kane additional research by Nico Haupt (sample quotes - full article is deep research w extensive citations)
“Pharmaceutical press releases are hitting the wires every day announcing multi-million dollar military contracts to create new vaccines including aerosols (potentially deliverable by airborne dispersion), nanotechnology, genetically modified pathogens, and the stockpiling of the same vaccines that have been harming Americans for decades – most notably BioPort's despicable anthrax vaccine.
With a bioterror attack on American soil having been virtually guaranteed by the Homeland Security Department, the reality of this translates into big bucks for the pharmaceutical industry.
FORCING EXPERIMENTAL VACCINES: JUNK SCIENCE
It's crucial to start from the beginning. On November 2, 1970, the FDA granted an anthrax vaccine license to MDPH (now BioPort) though neither had conducted a controlled field trial. The data presented in support of the vaccine made a mockery of science for three reasons.
• The study cited was of a completely different strain of vaccine made by Merck, Sharp, & Dohme.
• The manufacturing process used in making the vaccine changed when MDPH took over.
• The ingredients used to make the vaccine were changed.2
So this license was a complete fraud. How can a vaccine be licensed based on the testing of an entirely different vaccine? The whole sordid, disastrous history of anthrax vaccine follows from this central episode.
How can we even discuss the resulting mountain of distortions and lies, when the original license granted was fraudulent? The only ethical, and logical answer is to ban this vaccine in perpetuity.”
Dec. 22, 2003 the US District Court for the District of Columbia ordered the military to stop using its soldiers as guinea pigs for dangerous experimental anthrax vaccines.
Just days before this ruling the Pentagon ordered a $29.7 million order of anthrax vaccine from BioPort, part of a $245.6 million contract, in anticipation of the reversal.
Army sergeant Rachel Lacey died from complications caused by the anthrax vaccine, according to an autopsy report from the Mayo Clinic in Rochester, Minnesota.4 This was not an isolated case; the anthrax vaccine is the likely cause of a pneumonia-like illness that may be reaching epidemic proportions in our military. what they call a “mysterious pneumonia cluster”
Despite strong evidence suggesting that the vaccine is causing soldiers to get sick, the Pentagon is denying the possibility.
October 2003, when 80.3 million of our tax dollars were spent in ordering VaxGen Inc. to develop an experimental anthrax vaccine. An additional $71.3 million was contracted to Avecia for 3 million doses of a new recombinant anthrax vaccine.
FDA Suddenly Approves Anthrax Vaccine by BioPort
This vaccine had not received FDA approval for inhalation anthrax in over 30 years, and they've held it up for 18 years since 1985, but the FDA suddenly experienced a change of heart in perfect time to reverse Judge Sullivan's historic ruling. In the FDA statement from December 30, 2003, they specifically state that their decision was based on studies from 1985, so no new supporting evidence has been presented.14 The FDA is showing its true colors here. It is notorious for conflict of interest, and much of that conflict comes from FDA ties to big pharmaceutical companies.
Just days later VaxGen was granted FDA fast track status to get their “next generation” anthrax vaccine to market.
NYPD announced it has undergone massive contingency exercises for the scenario of a chemical or biological attack in Manhattan. Scenarios may include mass dissemination and administration of vaccines and quarantining people in their own homes. The city has also recently changed its health codes to detain anyone health officials suspect of having been exposed to a deadly infectious pathogen.
Nanotechnology and Gene Shuffling – New Insanity
BioSante Pharmaceuticals has just announced a subcontract with DynPort- a subsidiary of CSC-DynCorp to develop anthrax vaccines using nanotechnology based alternative delivery systems.25 Nanotechnology involves building new structures at the molecular level. Atoms are individually moved, one at a time, to create new molecules, some of which do not occur in nature.
The National Institute of Allergy and Infectious Disease NIAID is funding $8.3 million to University of Maryland researchers to develop an oral anthrax vaccine. They hope to provide vaccination with 2 doses of genetically engineered oral vaccine
The World Health Organization WHO is promoting aerosolized vaccines for measles and rubella. Clinical trials are planned for this year and could be completed by 2007. The two systems the WHO has presented for delivery of the vaccines are jet nebulizers and ultrasonic nebulizers.
Scarlet Cloud Drills Point to Illegal Aerosol Vaccine Solution
The story of a secret cabinet level experiment named Scarlet Cloud was reported by Suzanne Malveaux of CNN's Washington Bureau on Dec. 28, 2003, and by Judith Miller in the New York Times on the same day. Miller wrote that officials stated the exercise “…showed that antibiotics in some cities could not be distributed and administered quickly enough and that a widespread attack could kill thousands” – the widespread attack was war-gamed as anthrax….
DARPA – the Defense Advanced Research Project Agency, gave Maxygen a $3.8 million contract in 1998, and a $7.7 million contract in 1999 (Maxygen reports the contract as $6.7 million)
Maxygen literature indicated that this was a three-year grant to use its proprietary MolecularBreeding ™ directed evolution technology to protect against a broad spectrum of Pathogens . Maxygen is involved in what they themselves call “ gene-shuffling ”, creating genetically modified vaccines working directly with DARPA.
The military asked Maxygen to develop aerosol-based vaccines that could be inhaled to ‘safeguard' people against a broad range of pathogens. Shaun Jones, the first director of DARPA's Unconventional Countermeasures program has said the value of many of their programs will not be known till they are tested on people.33
“Scarlet Cloud” was a tabletop exercise that eerily resembles the mass emergency drill on October 24 through 26 of 2000, which practiced rapid response for a then-hypothetical scenario: an airliner being crashed into the Pentagon. Check the army's own website for the details. 30)
Military Mandate Anthrax Vaccines
Resource Directory Anthrax
You can also scroll down below this section for advice on what to do if you are facing deployment and are being ordered to take the vaccine, a chronology documenting the history of this vaccine, adverse reactions troops and veterans have reported, a full symptoms list taken from VAERS reports and medical reports, and more resources.
1. The FDA's new label (2002) on the anthrax vaccine admits to a systemic averse reaction rate of between 5% and 35% - whereas previously, the DoD claimed it was a mere .02%. Since then, the GAO (Government Accounting Office, a watch-dog agency) has come out with a new report estimating the systemic adverse reaction rate is probably as high as 85%. In addition, it is known that women have twice the adverse reaction rate as men. The GAO also issued a 2001 report that of the Guard and Reserve units forced to take the vaccine, fully 25% of the pilots resigned or obtained transfers out of their units rather than take the vaccine and jeopardize their civilian flying careers.
2. The anthrax vaccine was never licensed for use against aerosolized anthrax, and thus was used on our troops illegally up until December, 2003. Just eight days after the Dec. 22, 2003 decision by federal Judge Emmett Sullivan's that orders to take the vaccine were illegal, the FDA issued a ruling that it was both legal and safe - based on animal data. See further developments in the lawsuits section.
3. The anthrax vaccine was originally licensed based on data from a different vaccine. The only safety/efficacy study ever done on human beings was done on that different vaccine. The FDA and DoD have also previously admitted that efficacy based on animal studies against inhalation is problematic because no proven correlate of immunity between animals and humans exists for anthrax infection.
4. The anthrax vaccine protocol originally called for 3 shots only; the change to a series of 6 shots with annual boosters was done with no foundation in research or fact. The label was actually changed to reflect the protocol then in practice; the protocol was not dictated by instructions on the label.
5. BioPort, the original manufacturer of the vaccine, changed its filtering and fermenting equipment in 1991 without notifying the FDA. The result was a 100-fold increase in the potency of the vaccine. It was used anyway. BioPort also produced non-sterile and contaminated vaccine and changed expiration dates on labels of some of the vaccine lots to portray false expiration dates. BioPort's was issued a new contract with the government to produce more anthrax vaccine in the week between Judge Sullivan's ruling and the FDA declaration that the vaccine is legal. Other companies such as VacGen have recently been awarded huge government contracts to manufacture a new anthrax vaccine.
6. A Kansas State University Study has definitely pointed to the anthrax vaccine as one of the major components of Gulf War Syndrome.
7. An adjuvant, or vaccine booster, called squalene is present in an unknown number of lots of the anthrax vaccine. Though its presence was long denied by the DoD, FDA testing eventually proved its presence in at least five lots, possibly more. The use. of squalene as an adjuvant for human beings is illegal in both the U.S. and Great Britain. It is known to be a primary cause of severe arthritis when injected.
8. Micoplasma is also present in the vaccine. Mycoplasma are the smallest of free-living organisms, and can reproduce outside of living cells. They can cause chronic inflammatory diseases of the respiratory system, urogenital tract, and joints. The most common human illnesses caused by mycoplasma are due to infection with M. pneumoniae, which is responsible for 10-20% of all pneumonias. This type of pneumonia is also called atypical pneumonia, walking pneumonia, or community-acquired pneumonia. Infection moves easily among people in close contact because it is spread primarily when infected droplets circulate in the air (that is, become aerosolized), usually due to coughing, spitting, or sneezing.@ (Source: Gale Encyclopedia of Medicine online) 31)
Anthrax Vaccine Producers
VaxGen
May 7, 2008 (CIDRAP News) – VaxGen Inc announced this week the sale of its experimental anthrax vaccine which the US government pulled the plug on in 2006—to Emergent BioSolutions, maker of the only US-licensed anthrax vaccine.
The Department of Health and Human Services HHS awarded VaxGen an $877 million contract in 2004 to produce 75 million doses of its second-generation anthrax vaccine for the Strategic National Stockpile. But HHS canceled the contract in December 2006, after problems with the vaccine's stability caused the company to miss a deadline for starting a clinical trial.
VaxGen's contract was the first awarded under Project BioShield a $5.6 billion program to procure medical defenses against the effects of chemical, biological, radiological, and nuclear weapons. The aim of the contract was to provide a vaccine that requires fewer doses than the existing vaccine and has minimal side effects.
The Los Angeles Times reported last December that a lobbying campaign by Emergent had been a factor in the HHS decision to cancel the VaxGen contract. Emergent has a second-generation anthrax vaccine candidate of its own, called rPA 102. Like VaxGen's vaccine, its active ingredient is a recombinant form of protective antigen (rPA), an anthrax protein. 32)
Emergent Biosolution formerly BioPort Labs
ROCKVILLE, Md.–(BUSINESS WIRE)–May 5, 2008–Emergent BioSolutions Inc. (NYSE: EBS) announced today that it has completed the acquisition of all assets and rights related to a recombinant protective antigen (rPA) anthrax vaccine product candidate and related technology from VaxGen, Inc. Recent improvements to the rPA vaccine, specifically related to stability, suggests that it is well positioned to be a leading candidate for an award under a request for proposal (RFP) recently issued by the U.S. Department of Health and Human Services (HHS). The vaccine candidate has completed one Phase 2 clinical study. This RFP is designed to meet the government's stated goal to procure 25 million doses of an rPA anthrax vaccine for the Strategic National Stockpile (SNS)
The company intends to manufacture this new rPA anthrax vaccine, as well as BioThrax, in its recently constructed, large-scale manufacturing facility at its Lansing campus. The acquisition of the rPA vaccine candidate further solidifies Emergent's well established franchise of anthrax countermeasures, which now includes
BioThrax(R), the only FDA-approved vaccine to prevent the infection of anthrax. Nearly 2.0 million men and women of the United States military have received the vaccine, and HHS has procured more than 28 million doses of BioThrax for the SNS
rPA 102, a recombinant anthrax vaccine candidate, which is composed of a purified protein with an alum adjuvant and is designed to induce antibodies that neutralize anthrax toxins
AVP-21D9, a human monoclonal antibody product candidate being developed as an intravenous treatment for patients who present symptoms of anthrax disease and
AIG, a polyclonal anthrax immune globulin product candidate, which is derived from human plasma from individuals who have been vaccinated with BioThrax.
“As the manufacturer of the only FDA approved anthrax vaccine, Emergent BioSolutions has a proven track record of delivering critical biodefense countermeasures to the U.S. Government. Given HHS's stated commitment to procure up to an additional 25 million doses of a recombinant anthrax vaccine for the Strategic National Stockpile, we felt this was the right opportunity for our company at the right time,” said Fuad El-Hibri, chairman and chief executive officer of Emergent BioSolutions.
The vaccine candidate, rPA 102, is based on a recombinant form of the protective antigen protein. This vaccine contains a purified protein (rPA) formulated with an alum adjuvant and is designed to induce antibodies that neutralize anthrax toxins. The vaccine candidate does not cause anthrax infection and is based on the pioneering work of the U.S. Army Medical Research Institute of Infectious Diseases USAMRIID. rPA 102 has been the subject of two research and development grants totaling approximately $100 million from the National Institute for Allergy and Infectious Diseases NIAID. In 2004, HHS awarded VaxGen an $877 million contract for delivery of 75 million doses of rPA 102. 33)
Iomai Corp
Apr 8, 2008 (CIDRAP News) – Iomai Corp., a biotechnology company that specializes in needle-free vaccines, announced today that it will receive a grant from the US Department of Defense (DoD) to fund preclinical development of a patch-based anthrax vaccine.
The 1-year grant to Iomai, based in Gaithersburg, Md., will be in the form of a $943,856 cost reimbursement from the US Army Medical Research and Material Command, according to a press release from Iomai.
Work on the vaccine will combine an antigen developed by Avecia Biologics, Ltd., based in the United Kingdom, with an Iomai adjuvant on a skin patch. Iomai said its next step would be to gauge the stability of the patch to see if it can be stored and shipped at room temperature.
The current licensed anthrax vaccine, made by Emergent BioSolutions Inc., must be refrigerated and is given as a six-shot regimen over 18 months. The US government has been seeking a second-generation product to replace anthrax vaccine adsorbed (AVA), developed in the 1950s, with hopes that a newer product would require fewer doses and produce minimal side effects. Some military personnel have objected to AVA because of reported serious side effects. 34)
Schools Developing [[Bioterrorism]] Vaccines
Newsday - September 7, 2003 by Julie Halenar
“University of Maryland School of Medicine has been chosen to lead a multi-school effort to develop vaccines to protect against bioterrorism, the school announced Thursday.
The Middle Atlantic region will receive a five-year, $42 million grant from the National Institute of Allergy and Infectious Diseases. Besides creating vaccines to guard against anthrax, smallpox and West Nile virus, they will study antibodies that could produce short-term protection….
Myron Levine of the University of Maryland School of Medicine will be in charge of the collaboration of 16 research institutions – such as Johns Hopkins University, University of Pennsylvania, University of Virginia, Georgetown University, George Washington University, West Virginia University and University of Pittsburgh. He will guide more than 60 scientists at three facilities in the region…
The other centers are Duke University, Harvard Medical School, New York State Department of Health, University of Chicago, University of Texas Medical Branch, University of Washington and Washington University in St. Louis.” 35)
BOOKS
Tom Heemstra, a former F-16 Fighter Squadron Commander, takes you deep into politically charged controversy and exposes the truth about the unsafe, untested, unethical and illegal anthrax vaccine. The Department of Defense’s mandatory Anthrax Vaccine Immunization Program (AVIP) resulted in illness and death - from a vaccine that was supposed to protect our military forces.
Heemstra, a former F-16 Fighter Squadron Commander, is speaking out around the country to promote the book and expose the problems behind the U.S. military's Anthrax Vaccine Immunization Program (AVIP). The book explores in depth the politically charged controversy that exposes an unsafe, untested, unethical and illegal vaccine, and contains information about both anthrax and the vaccine not generally known to the public. 36)
Bioport client GlobalOptions 'Crisis management services' ~ Blackwater meets James Bond
“GlobalOptions boasts an extraordinary stable of intelligence and law enforcement professionals, legal support, public relations specialists, and veterans of America’s most elite military units. Never before have all of these services been assembled under a single umbrella.” 37)
Resources
Anthrax Vaccine - Military Vaccine Resource Directory.
https://web.archive.org/web/20070618184701/http://www.mvrd.org/showpage.cfm?ID=2
Dirtbag Trackers
October 12, 2001 THE OVERTHROW OF THE AMERICAN REPUBLIC, Part Three by Sherman H. Skolnick
THE ANTHRAX COMMISSARS -
Could the Anthrax Mailings Be Military-Industrial Espionage? A Special Report By Leonard G. Horowitz, D.M.D., M.A., M.P.H.* Outstanding timeline
*ANOTHER LEFTOVER SCANDAL FROM CLINTON BACKGROUND OF ANTHRAX VACCINE MANUFACTURER BIOPORT*
By Reed Irvine and Cliff Kincaid - May 22, 2001
Admiral Crowe provides a cover of respectability to a company that has some rather curious foreign connections. Bioport was created by Crowe and his partners out of another company called Intervac. Crowe owns 22.5 percent of Intervac shares, although it’s been reported that he hasn’t “invested a penny” in the venture. Rosenberg’s investigation also determined that 30 percent of Intervac shares are owned by Nancy El-Hibri, a mother and homemaker in suburban Maryland and the rest of the company is in the hands of “I&F; Holdings,” a company directed by Nancy El-Hibri’s father-in-law, Ibrahim El-Hibri, a Venezuelan citizen, and her husband, Fuad El-Hibri, a German citizen of Lebanese descent. Rosenberg said that Fuad El-Hibri appears to be the real day-to-day director of Intervac and is listed by Dun & Bradstreet as the “chief executive” of Bioport. 40)
ANTHRAX and VESTED INTERESTS 100% Chance Of Bio Attack In The US!” say the networks. Hmmm
The Black Vault - largest collection of FOIA documents published for public use
*Medical aspects of Biological Warfare*
Published 2007 by the Office of The Surgeon General Department of the Army, United States of America and US Army Medical Department Center and School Fort Sam Houston, Texas - Borden Institute Walter Reed Army Medical Center Washington, DC 42)
Whitney Webb. September 11, 2020 *A Killer Enterprise* 43)