This is an old revision of the document!
BioNTech
Development of mRNA Vaccine Technology
On October 16, 2019, Nature published an article describing BioNTech's successes in testing experimental immunotherapy vaccines for cancer, and suggested the progress could be applied to solve problems like rabies and pandemic influenza.1)
Clinical Trials Pfizer FOIA
BioNTech RNA Pharmaceuticals GmbH Confidential Interim Clinical Study Report
BNT162-01 Page 1 of 1 Version: 2.0 Date: 26 Nov 2020 3)
16.1.13
List of sponsor personnel who materially affected the trial conduct The following table presents a list of sponsor personnel who materially affected the trial conduct. The full list of all involved sponsor personnel is maintained in the trial master file (looking for it)
- Name Role
- Özlem Türeci, MD Chief Medical Officer (Sponsor’s medically qualified person) b
- Svetlana Shpyro, MD Medical Expert
- Dr. Stefan Liebscher Biostatistician (External BioNTech Consultant)
- Sean Murphy Biostatistician (External BioNTech Consultant)
- Dr. Christopher Marshallsay Head Scientific-Medical Writing a
- Dr. Frans van Huizen Senior Scientific / Medical Writer
- Dr. David Langer Clinical Study Manager
- Dr. Stefanie Bolte Clinical Study Manager
- Martin Bexon, MD Medical Monitor (External BioNTech Consultant)
- Amélie Caneparo Pharmacovigilance Manager
- Claudia Müller Clinical Data Manager (External BioNTech Consultant)
- Dr. Tania Palanche Senior Clinical Data Manager
a) Document owner fulfills the requirements of the author according to ICH E3.
b) Corresponds to the medical officer defined in ICH E3. 4)

Destroying BNT162b2 Placebo Group In 180 days
Random sample - vaccine dates are squished together above dose type BNT162b2 or Placebo.
5)
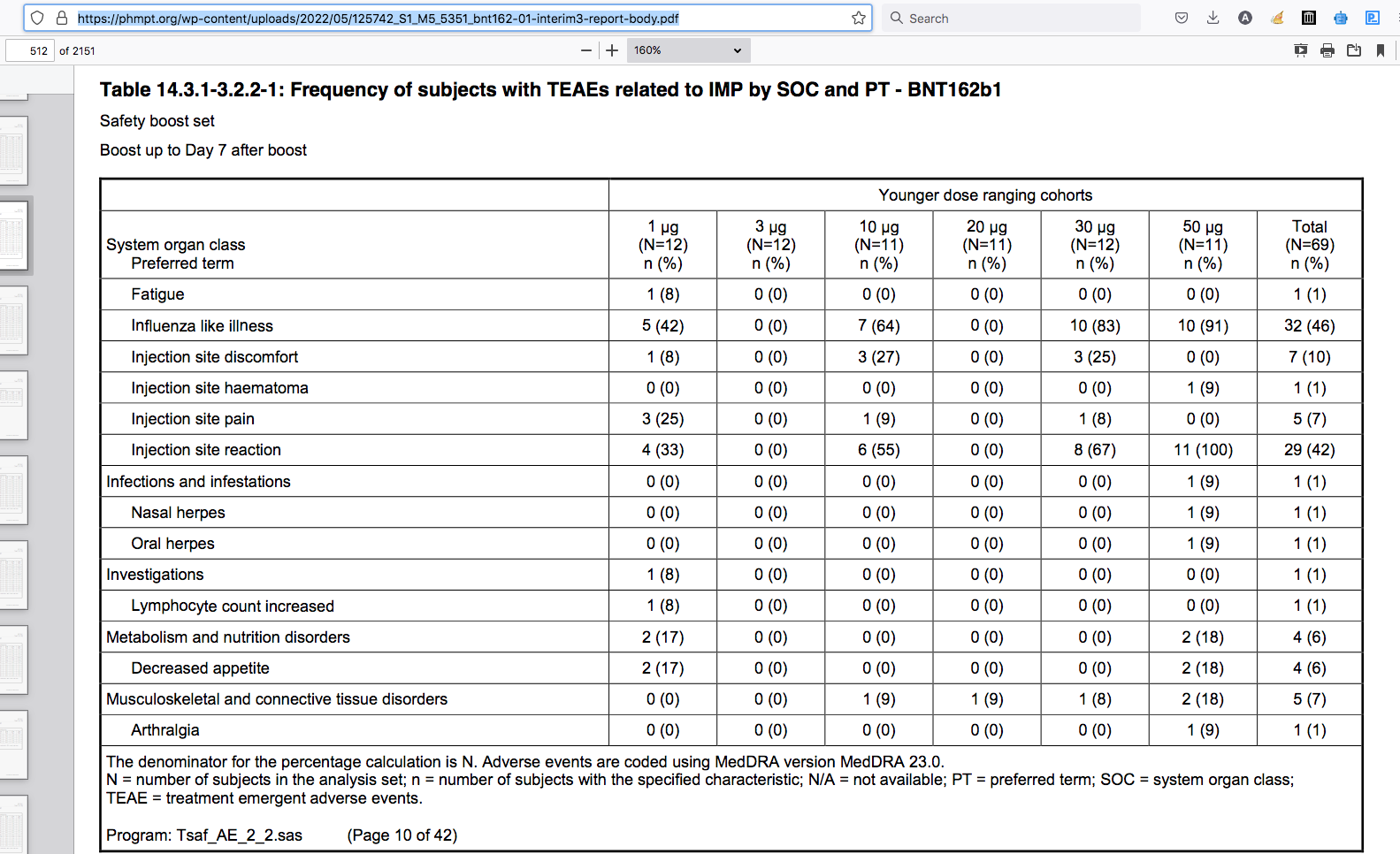
Adverse Events
BioNTech RNA Pharmaceuticals GmbH / Tables - 6. Snapshot All TLFs Final 1.0 BNT162b1 BNT162-01 Created on 20NOV2020 Staburo GmbH. Based on clean SDTM data received on 03NOV2020. Data cut-off: 23OCT2020. Table 14.3.1-3.2.2-1: Frequency of subjects with TEAEs related to IMP by SOC and PT - BNT162b1
pdf page 512/21516)
REFERENCES
page 140/2151 - REFERENCES7) Boraschi D, Del Giudice G, Dutel C, et al. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine 2010; 28(21): 3627-31.
Destexhe E, Prinsen MK, van Schöll I, et al. Evaluation of C-reactive protein as an inflammatory biomarker in rabbits for vaccine nonclinical safety studies. J Pharmacol
Toxico. Methods 2013; 68: 367-73.
Doener F, Hong HS, Meyer I, et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine 2019; 37: 1819-26.
Kamphuis E, Junt T, Waibler Z, et al. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 2006; 108: 3253-61.
Mulligan M, Lyke KE, Kitchinet N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586(7830): 589-93.
Taylor DN, Treanor JJ, Sheldon EA, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine 2012; 30: 5761-9.
Tsai MY, Hanson NQ, Straka RJ, et al. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005; 145: 323-7.
US FDA Guidance for Industry - Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials.
Walsh EE, Frenck R, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020; 383(25): 2439-50.8)